April 26, 2022
Holistic strategy for UDI compliance
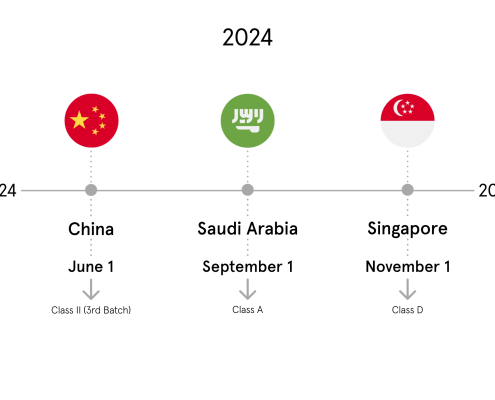
Evolving regulatory requirements are one of the crucial challenges for the life sciences industry. Complying and keeping up with UDI regulatory requirements is a very daunting and challenging task, but necessary to ensure you maintain your presence in current UDI markets, as well as it gives you the opportunity to be prepared for upcoming, unknown regulations.
To keep up, it’s critical to develop a company-wide and global strategy for centrally managing UDI product data using process automation to achieve and ensure global UDI compliance.
But how do you as a responsible person for UDI in your medical device company maintain compliance while adopting new business models that are more patientcentric and service-based?
As UDI compliance becomes more complex, the time is now to automate process the master data management and regulatory submission.
Therefore, we recommend watching the webcast from SAP and p36 to be the first to hear how to develop a company-wide and global strategy for centrally managing UDI product data using process automation to achieve and ensure global UDI compliance within your company.
We will discuss the challenges and opportunities of UDI compliance and how technology can help:
- Manage the complexity of UDI compliance across many countries in one platform
- Maintain efficient data government processes
- Ensure global UDI compliance in the long-term
We hope you find this discussion insightful!