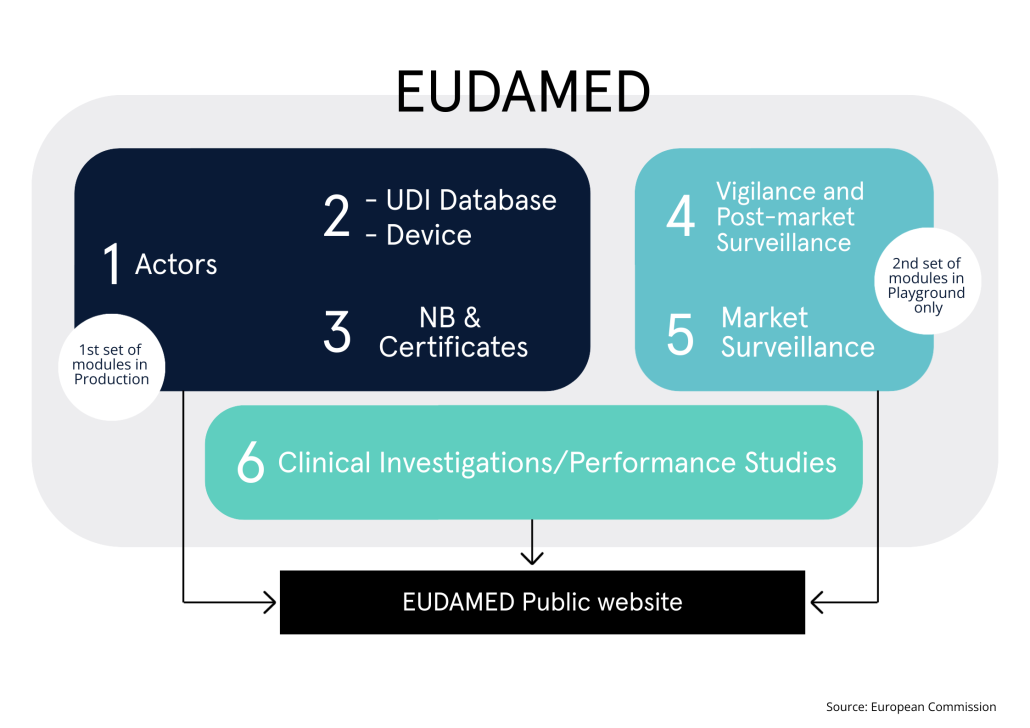
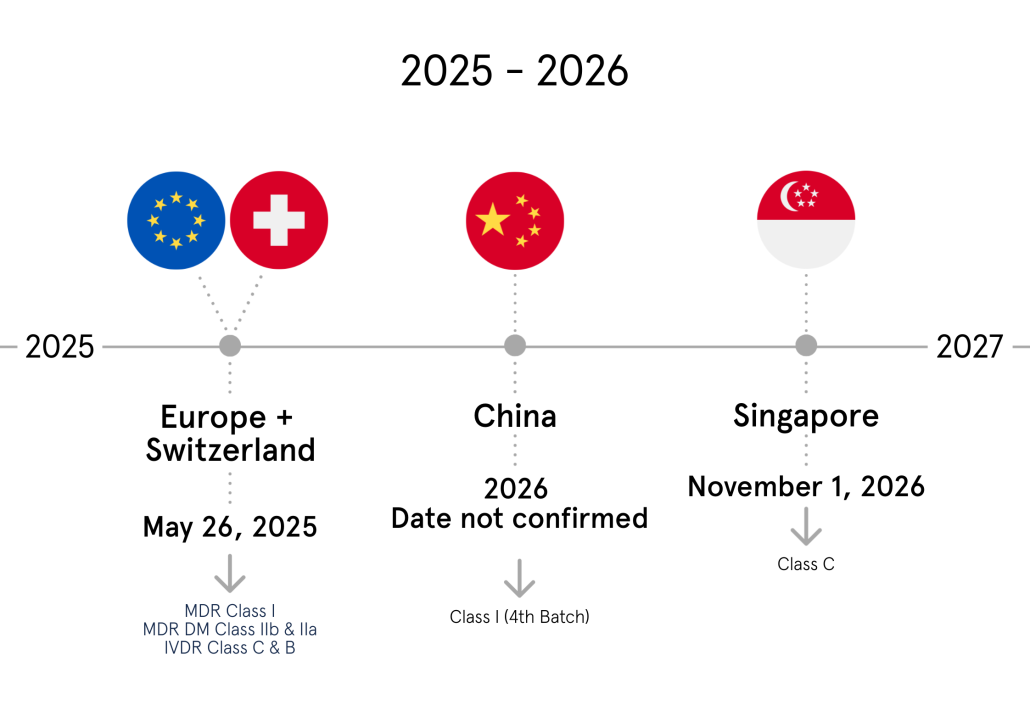
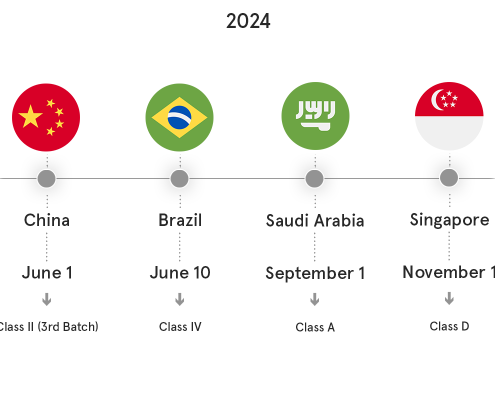
The turn of the year has brought us, unexpectedly, totally refreshed appeal of UDI Portal, now known as ‘Medical Device Safe Bookstore’.
From UDI and M2M data exchange perspective, main change was API host address replacement as the database was migrated into new space. Obviously, all functionalities and datasets were upheld.
We observe huge step forward from MFDS side as the team responsible for ‘emedi’ is way more open in communication and is constantly seeking upgrades in provided databases, making life of manufacturers and 3rd party providers like p36 easier. For example, in February they announced a campaign of gathering ideas for improvements for all services, we encourage you to share it with local affiliates and us to actively support the IMDIS improvements.
Moreover, there are whole new documents, namely the whole rebuilt User Guide that puts much more light on the requirements that many companies have struggled with!
 https://www.p36.io/wp-content/uploads/2024/03/atrify_Partnership_2024.jpg
1254
2400
Michael Teufel
https://www.p36.io/wp-content/uploads/2023/03/p36_logo-1.png
Michael Teufel2024-03-27 15:30:062024-03-27 15:45:01p36 GmbH and atrify GmbH, a 1WorldSync Company, Announce Partnership on Global UDI Compliance
https://www.p36.io/wp-content/uploads/2024/03/atrify_Partnership_2024.jpg
1254
2400
Michael Teufel
https://www.p36.io/wp-content/uploads/2023/03/p36_logo-1.png
Michael Teufel2024-03-27 15:30:062024-03-27 15:45:01p36 GmbH and atrify GmbH, a 1WorldSync Company, Announce Partnership on Global UDI Compliance