May 12, 2022
Regulatory Update 04/2022
Dear reader,
Complying and keeping up with UDI regulatory requirements is a very daunting and challenging task, but necessary to ensure you maintain your presence in current UDI markets, as well as it gives you the opportunity to be prepared for upcoming, unknown regulations.
As usual, we would like to provide you with an overview on the current UDI requirements worldwide with our newsletter, to help you take the next steps towards your global UDI compliance.
Furthermore, we would like to announce our webcast together with SAP on how “UDI Compliance can be Managed and Scaled”.
We would like to point out that the information provided has been selected very carefully by us. Nevertheless, we cannot guarantee or assume liability for the timeliness, accuracy, completeness or omission of the published information. Further information can be found in our disclaimer.
Stay healthy and confident!
Best regards,
Your p36 team
Content
UDI in Europe and Worldwide
- China I NMPA (CUDID)
- South Korea I MFDS (IMDIS)
- Saudi Arabia I SFDA (SAUDI-DI)
- Brazil I ANVISA
- Egypt I EDA
- Singapore I HSA (SMDR)
UDI in Europe and Worldwide
More and more regulatory authorities are coming up with their own UDI regulations – such as Brazil, Singapore and Taiwan, just to name a few markets.
Along with these known and unknown requirements, it is becoming increasingly important for medical device companies to establish a global strategy for UDI and the management of multiple data worldwide. The following section therefore provides a brief overview of what we consider to be the most important upcoming changes worldwide for you as a medical device manufacturer.
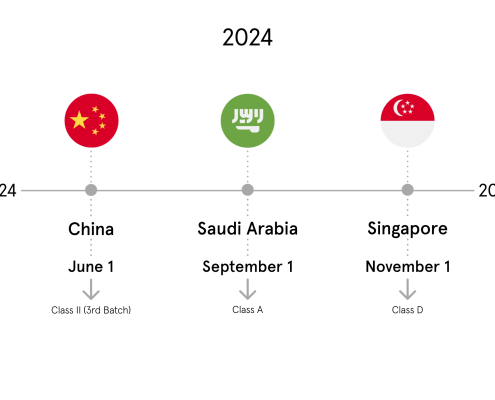
China I NMPA (CUDID)
In September 2021 the NMPA announced the official deadline for the registration of medical devices in the Chinese UDI database CUDID.With this decision the remaining medical devices from Class III have to be registered by June 1, 2022.
South Korea I MFDS (IMDIS)
As the last deadline for Class I medical devices in South Korea expires on July 1, 2022, it is recommended to prepare the products of this class for submission to the South Korean database IMDIS.
Saudi Arabia I SFDA (SAUDI-DI)
As we informed in our previous newsletter, the first deadline for medical devices in Saudi Arabia will be September 1, 2022. Products of Class D, C and B must be registered by this date.
Brazil I ANVISA
At the end of last year, the ANVISA published its official guideline on UDI requirements in Brazil. As expected, the UDI requirements are aligned with the requirements of the IMDRF and are effective since January 10, 2022.
The compliance deadlines for medical devices, depending on their risk class, are as follows:
- Risk class IV: July 10, 2024
- Risk class III: January 10, 2025
- Risk class II: January 10, 2026
- Risk class I: January 10, 2028.
Egypt I EDA
The egyptian regulatory authority EDA recently published its own guidance for their UDI requirements on medical devices. Although no exact deadlines are known yet, the requirements that have to be met for UDI in Egypt seem to be aligned with the IMDRF guidance with local adaptations.
As soon as we have more information regarding the deadlines and implementation, we will inform you on our social media channels at short notice.
Singapore I HSA (SMDR)
Last year, Singapore published its final guide on UDI requirements on medical devices.
Phase 1 will start by November 1, 2022 with high risk implantable medical devices, such as coronary stents, orthopaedic joint replacement implants and intraocular lenses.
Webcast: UDI can be Managed and Scaled
Benefit from a Holistic Strategy to Achieve UDI Compliance
Learn how process automation can help safeguard global UDI compliance
Evolving regulatory requirements are one of the crucial challenges for the life sciences industry. And UDI compliance is going to become more complex; the time is now to automate the process of master data governance and regulatory submissions.
To keep up, it’s critical to develop a company-wide and global strategy for centrally managing UDI product data using process automation to achieve and ensure global UDI compliance.
But how do you maintain compliance while adopting new business models that are more patientcentric and service-based?
Be the first to hear from SAP and p36 how to develop a company-wide and global strategy for centrally managing UDI product data using process automation to achieve and ensure global UDI compliance.
We will discuss the challenges and opportunities of UDI compliance and how technology can help:
- Manage the complexity of UDI compliance across many countries in one platform
- Maintain efficient data government processes
- Ensure global UDI compliance in the long-term
We hope you find this discussion insightful!
Benefit from a Holistic Strategy to Achieve UDI Compliance
Speakers
- Phil Foust, Global Partner Business Development, Life Sciences & Healthcare Ecosystems, SAP
- Patrick Pfau, Managing Director, p36
We hope this newsletter has given you a helpful overview on the current UDI requirements all over the world. Please keep in mind that timelines may change.
In case of any updates, we will inform you about it on our social media accounts at short notice.
Do you have any questions or suggestions regarding our Newsletter?
We appreciate to hear from you.
Disclaimer
p36 assumes no liability or responsibility for damages or consequential damages of any kind arising from the use of the information provided.
All information, statements and data provided are non-binding and may be changed or deleted in whole or in part by p36 at any time without prior notice.
All information relates to the current state of knowledge of p36 at the time of publication. We endeavor to select all information provided with care and to update it as necessary, but all information/future-oriented statements are subject to various risks and uncertainties that may cause actual results to differ materially from expectations. Readers are therefore cautioned not to place undue reliance on such information/future-oriented statements.
The information may not be incorporated into any contract.
p36 does not warrant or assume liability for the timeliness, accuracy, completeness or omissions of the information published. The information does not constitute a commitment, promise or legal obligation. All information does not constitute advice in the legal sense. p36 assumes no responsibility for the legal force and legal admissibility of the content and information. The information is provided without warranty of any kind, either express or implied.