November 30, 2023
Dear readers,
we are returning with a portion of regulatory news from across the globe.
Some authorities seem to speed up the UDI databases implementation process whereas European Commission again leaves us with more uncertainties than before.
Below you will find most appealing information we received since our last issue of newsletter in June. The visualization of the UDI timeline accompanies the quoted news. At the end of the newsletter, you will find a brief summary of the most important aspects.
As usual, we would like to point out that the information provided has been selected very carefully by us. Nevertheless, we cannot guarantee or assume liability for the timeliness, accuracy, completeness, or omission of the published information. Further information can be found in our disclaimer.
Stay healthy and confident!
Best regards,
Pascal Appel
UDI Content Package Manager
UDI Rules
Europe I EC (EUDAMED)
Last time we happily announced the refreshed timeline for EUDAMED hoping soon we will all see the launch of the database.
Unfortunately, one more time, a postponement was announced. Latest official statement from EU Commission surprised us all as most part of the industry expected full release in 2025.
Below we present the latest official timeline that sights as long as 2029:
- Q2 2024: End of MVP development (for 5 modules only; CI/PS excluded)
- Q1 2025: End of 5 modules audit
- Q2 2027: Finishing of further development of CI/PS module with its audit. ‘Fully operational EUDAMED database launch’
- Q4 2027: Partially mandatory data registration period starts
(*6 months after fully operational) - Q2 2029: EUDAMED becomes mandatory for all Classes of medical devices
(*2 years after fully operational)
Moreover, one more self built bump on the road for EUDAMED could be Master UDI MDR amendment announcement. It will be applicable from Q3 2025 for contact lenses. Currently there is no detailed technical specification for this yet another type of UDI.
We keep a close eye on the topic, as it could turn the UDI world upside down for both EU Commission and concerned manufacturers.
USA I FDA (GUDID)
In August U.S. FDA updated the fields released in the public GUDID database. It covered latest value list of GMDN Term Codes along with their status (active/obsolete).
For devices registered only with FDA PT Codes there are now also publicly visible corresponding GMDN codes. It significantly enhances search and retrieval capabilities for GUDID and structuring of enclosed datasets.
Attention:
We would like to remind that all applicable changes to the UDI relevant data should be submitted in 10 business days when it does not appear on the label of the device or not later than the date a device is first labelled with the changed information.
Please check your existing datasets if there are no obsolete terms assigned if not done already.
Saudi Arabia I SFDA (Saudi-DI)
The peak of workload for submission of UDI data to Saudi-DI fell on September 2023, unsurprisingly overlapping with Class D, C and B submission deadline.
It caused significant overload of the database and right now it’s only partially operational making it impossible to process data as expected.
Nevertheless, some improvements were implemented- bulk upload of UDI data was enabled through Excel files templates that correspond to different types of medical devices.
It speeds up the submission process significantly.
Australia I TGA (AusUDID)
Currently Australian TGA looks like the most robust and busy among all UDI regulators and we are expecting true breakthrough in implementation of AusUDID!
Voluntary compliance is announced to be opened as soon as Q2 2024!
Currently Pre-production environment is open for registration and testing for all interested parties. Data dictionary is soon to be publicly available. Most important fact is that TGA plans to launch AusUDID as truly complete system with different data submission interfaces to meet needs of industry. Officially there will be possibility to interchangeably use manual submission interface for single device, and both bulk upload or direct M2M data transmission for multiple devices at once.
Moreover, this year we can expect that TGA will officially extend the deadlines that would give manufacturers time to obtain an EU MDR certificate before the end of the EU MDR transition on December 31, 2028, and for sponsors to use the MDR certificate to apply to the TGA before July 1, 2029.
Currently there is no change in UDI implementation timeline on the devices themselves but all UDI topics are under discussion.
It is confirmed that we can expect relevant regulations covering all aforementioned subjects until the end of this year.
Switzerland I Swissmedic (SwissDAMED)
In June a pilot phase for SwissDAMED was conducted successfully with first (Actor) module of the database.
Is seems that the project is on a good track and beginning 2024 the on-boarding for the productive version of database will be open.
Later in Q3 2024 Swissmedic will deliver Minimum Viable Product enabling voluntary registration of medical devices.
Nevertheless, strong dependence on EUDAMED availability and MDR/IVDR transitional period mean that mandatory usage of SwissDAMED will not be in place before the former database will be fully operational.
While waiting for full technical specifications from Swissmedic we can share our concerns that currently there is no M2M interface planned and bulk upload operations are still in scoping phase leaving users only one alternative- manual entry for each device which would significantly raise workload on manufacturers side.
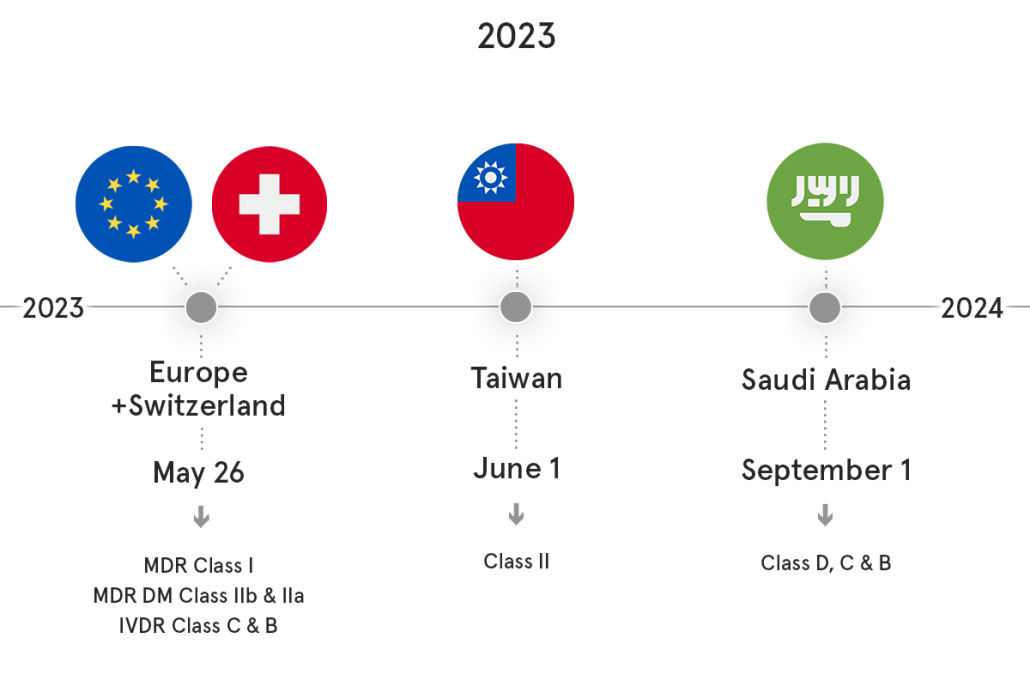
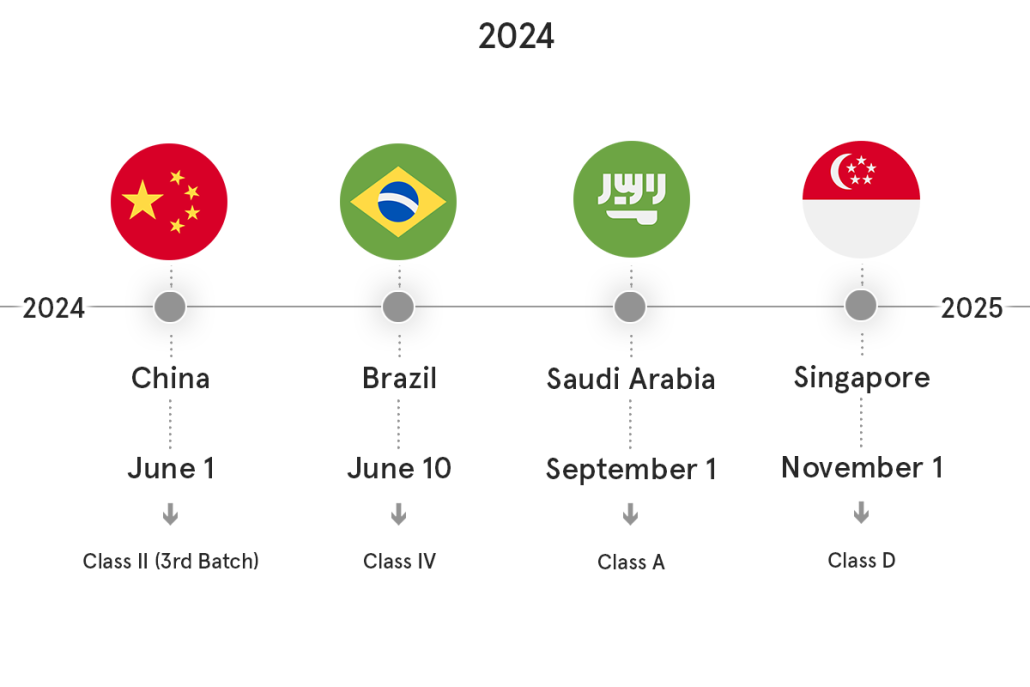
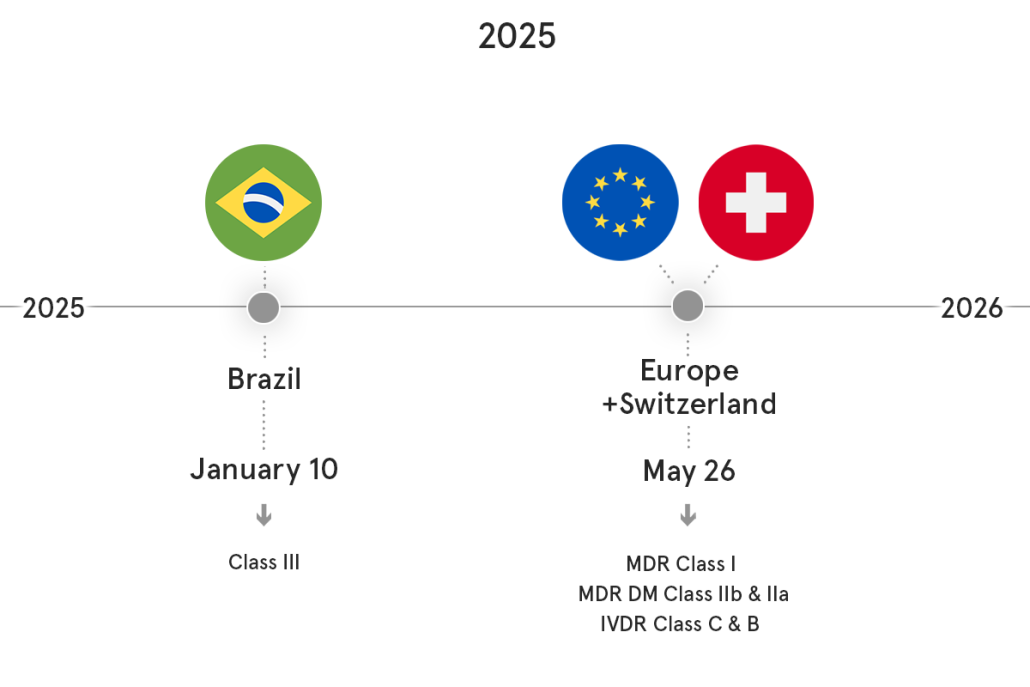
UDI Compliance Calendar
UDI in a Nutshell
UDI landscape has brought us significant updates last months:
- In Europe, once again EUDAMED was postponed, database is starting to become mandatory not earlier than 2027 according to current timeline.
- On the contrary FDA is working hard to extend capabilities of the database, making it more useful and user friendly providing searching improvements.
- Saudi Arabia and Australia are working hard on bringing functional databases for the industry soon!
- Switzerland even though mostly dependent on EUDAMED release, will launch pilot version next year.
Overall, UDI compliance requirements for labelling are maintained and databases development topics seem lively proceeded by authorities worldwide!
As always global regulators prepared new UDI requirements and shifted deadlines. This is not a one time project, but more a long term and ever changing global process right now. We are sure that UDI will bring challenges throughout coming and next years. That is why we at p36 will continue to monitor global market requirements for UDI as before and continue to work on the best possible solution for our customers so they can focus on their core business.
We hope this update has given you an useful overview of the current UDI requirements worldwide. If you want to be informed about current regulatory requirements before anyone else, you should subscribe to our Regulatory Newsletter.
Please note that timelines may change.
As usual, we will inform you of any updates at short notice via our social media channels.
If you have any questions or suggestions regarding our newsletter, we look forward to hearing from you.

Pascal Appel
p36Disclaimer
p36 assumes no liability or responsibility for damages or consequential damages of any kind arising from the use of the information provided. All information, statements and data provided are non-binding and may be changed or deleted in whole or in part by p36 at any time without prior notice.
All information relates to the current state of knowledge of p36 at the time of publication. We endeavor to select all information provided with care and to update it as necessary, but all information/future-oriented statements are subject to various risks and uncertainties that may cause actual results to differ materially from expectations. Readers are therefore cautioned not to place undue reliance on such information/future-oriented statements. The information may not be incorporated into any contract.
p36 does not warrant or assume liability for the timeliness, accuracy, completeness or omissions of the information published. The information does not constitute a commitment, promise or legal obligation. All information does not constitute advice in the legal sense. p36 assumes no responsibility for the legal force and legal admissibility of the content and information. The information is provided without warranty of any kind, either express or implied.