January 13, 2022
Webinar: Globale UDI-Anforderungen mit der richtigen Strategie umsetzen (German)
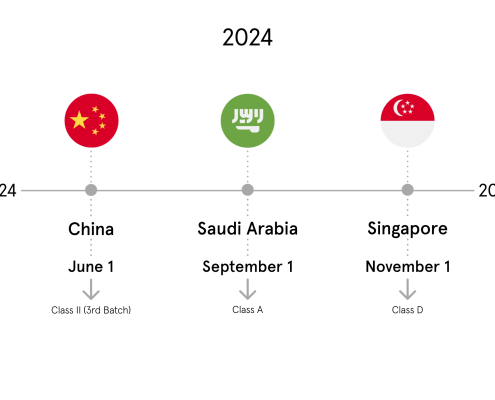
Medical device manufacturers around the globe are facing the challenge of meeting various UDI requirements in several markets worldwide in order to be able to distribute their products in the respective markets.
As part of the Life Sciences Alliance webinar series, we are hosting a webinar on “UDI: Implementing Global Requirements with the Right Strategy” together with our partner DHC. The webinar will take place on Friday, February 04, 2022 from 10:00 – 10:45 am (CET) and is free of charge. The webinar will be held in German.
Within the event, our Managing Director Patrick Pfau will talk about how medical device manufacturers can strategically prepare themselves most efficiently for the increasing global UDI requirements and thus ensure their global UDI compliance in the long term.
The contents of the webinar at a glance:
-
Overview of global UDI requirements
-
Strategic considerations and system requirements for a global UDI setup
-
System demo: Data preparation and submission to various regulatory agencies using the UDI Platform as an example.
Find more information here.